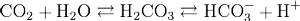Lesson 1: Solutions and pH
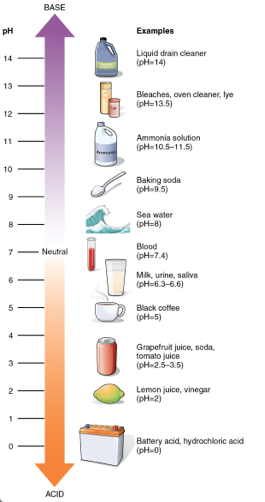
All liquids are either acidic or basic. If the molecules in water release more hydrogen (H+), than hydroxide (OH-), the solution is acidic. If they release more hydroxide the solution is basic. The pH of a solution is a measurement of concentration of hydrogen on a scale of 1 to 14. From 1-7 are the acids (lower number corresponds to higher acidity), while 7 is neutral. A pH above 7 is considered basic. The diagram below shows the pH values for common solutions.
Lesson 2: Formation of acid via industrial processes
Part 1: Formation of nitric and nitrous acids
Pure rainwater is normally slightly acidic, with a pH of about 5.6. As discussed in the introduction, acidity in precipitation has increased due to the presence of compounds containing sulfur and nitrogen. These are produced during the combustion of fossil fuels. First, we will look at how nitric acid is formed from nitrogen oxides.
In this reaction, nitrogen and oxygen react at high temperatures to form nitric oxide:
N2(g) + O2(g) → 2NO(g)
From here, nitric oxide reacts repeatedly with oxygen in the atmosphere to form nitrogen dioxide:
2NO(g) + O2(g) → 2NO2(g)
To form acid rain, nitrogen dioxide dissolves in water to create equal proportions of nitric acid and nitrous acid:
2NO2(g) + H2O(l) → HNO2(aq) + HNO3(aq)
Part 2: Formation of sulfuric acid
Sulfuric acid is one of the strongest in nature. Industrial processes leads to an increase of sulfuric acid in the environment.
For example, extracting metals such as zinc and copper from sulfide ores results in the release sulfur dioxide gas:
2ZnS(s) + 3O2(g) → 2ZnO(s) + 2SO2(g)
The gas can then react with water to form sulfurous acid: A subsequent reaction with oxygen gas forms sulfuric acid.
SO2(g) + H2O(l) → H2SO3(aq)
A subsequent reaction with oxygen gas forms sulfuric acid:
2H2SO3(aq) + O2(g) → 2H2SO4(aq)
Also, if you're having trouble making sense of these equations, click here for a quick explaination.
Lesson 3: How this applies to the Adirondack Park
As the EPA puts it, "acid rain isn't just a regional problem; it is a global problem."
When nitrogen and sulfur are released into the environment, they mix readily with water in the air. These pollutants are then dispersed by the wind and deposited elsewhere via rain and snow.
Here is a very helpful animation of this phenomenon: http://www.epa.gov/acidrain/education/site_students/acid_anim.html
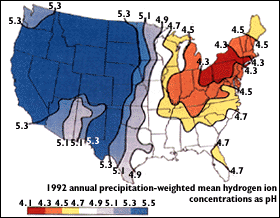
Due to wind patterns, much of these pollutants are deposited in northeast America, and especially in the Adirondack Park. This means that gases released in China can react to form acidic compounds that fall in the Adirondacks. Unfortunately, natural buffering systems cannot keep up with the amount of acidic deposition caused by human activity today.
The figure below gives a good glimpse of wind patterns, and acid deposition patterns in the United States.
Lesson 4: Buffering
Normally, naturally acidic deposition is neutralized by soils. This is called the buffering effect, a process by which acids and bases are neutralized. Buffering occurs in inorganic matter as well as in plants and animals. As an example, carbonic acid is an excellent buffering agent. Carbonic acid is formed when CO2 combines with water. The following reaction illustrates this phenomenon:
The acid then dissociates into H+ and bicarbonate ions (bicarbonate, or HCO3-, is a negatively charged ion similar to the previously discussed OH- species). In the case of acid influx, the bicarbonate species can neutralize the H+ ions, and the reaction runs in the opposite direction to reform H2CO3.
Activity: Self Quiz
Instructions: Click on the best answer to the questions below.
Question 1: Which of the following is TRUE about rainwater?
a) human activity leads to rainwater with a pH of ~5.6
b) rainwater is naturally neutral
c) rainwater is naturally acidic
d) rainwater is made more acidic by human activity
Question 2: Where is acid rain most prevalent in the United States?
a) acid rain is equally dispersed in the US
b) the northeast
c) the west coast
d) the midwest
Question 3: Which of the following is TRUE?
a) if more H+ ions are present than OH-, the solution is considered basic
b) in acidic conditions, only hydride ions (H+) are present
c) a neutral solution is 50% hydroxide and 50% hydride
d) a neutral solution is mostly water with a very small concentration of H+ and OH- present